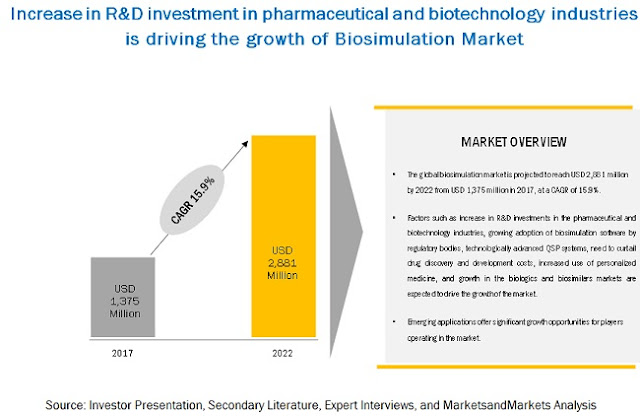
Factors such as increase in R&D investments in the pharmaceutical and biotechnology industries, growing adoption of biosimulation software by regulatory bodies, technologically advanced QSP systems, need to curtail drug discovery and development costs, and growth in the biologics and biosimilars markets are driving the growth of the market.
Market Revenue Growth Analysis:
The global biosimulation market is projected to reach USD 2,881 billion by 2022 from USD 1,375 billion in 2017, at a CAGR of 15.9%.
Market Segmentation Analysis:
Based on product & service, the biosimulation market is segmented into software and services. The biosimulation software segment is expected to account for the largest market share in 2017. The high growth in this segment is attributed to the growing adoption of biosimulation software by pharmaceutical and research organizations and the increasing R&D investment for pharmaceutical research.
Growth in the global biosimilars market is mainly driven by factors such as the growing pressure to curtail healthcare expenditures, growing demand for biosimilars due to their cost-effectiveness, rising incidence of various diseases, increasing number of off-patented drugs, positive outcomes in ongoing clinical trials, and rising demand for biosimilars in different therapeutic applications such as rheumatoid arthritis and blood disorders. As there is very little success in the R&D of new chemical entities, pharmaceutical companies are trying to find new applications for their existing drugs. As toxicity and other vital parameters of drug safety are already tested, biosimulation technologies are used to confirm the hypothesis of using the drugs for a new indication or disease.
Geographic Growth Analysis:
The biosimulation market is segmented into four major regions, namely, North America, Europe, Asia, and the Rest of the World (RoW). The dominance of the North American market is attributed to factors such as growth in the biotechnology and pharmaceutical industry, a large number of ongoing drug development processes, increased use of personalized medicine, and increasing R&D expenditure by pharmaceutical and biotechnology companies.
Download PDF Brochure:
https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=838
Key Players:
Certara (US), Simulations Plus (US), Dassault Systèmes (France), Schrödinger (US), ACD/Labs (Canada), Chemical Computing Group (Canada), Physiomics (UK), Evidera (US), In silico biosciences (US), INOSIM Software (Germany), Insilico Biotechnology (Germany), LeadInvent Technologies (India), Rosa (US), Nuventra Pharma (US), and Genedata (Switzerland).
Recent Developments:
In April 2017, Simulations Plus signed Distributor Agreement with Quantum Bio Solutions (Q-Bio) (South Korea) To strengthen its relationships with South Korean companies and universities.
In June 2017, Simulations Plus acquired DILIsym Services, Inc. (“DILIsym”) (US). This enabled the company to expand its product portfolio in simulation software and consulting services of drug-induced liver injury.
In December 2015, Certara acquired XenologiQ (UK) To strengthen its product portfolio in mechanistic pharmacology and modeling and simulation.


